How Did Niels Bohr Describe Electrons in His Atomic Model
Bohrs most significant contribution was the quantisation of the model. The transition requiring the greatest energy change is.
Bohr S Atomic Model Overview Importance Expii
It also describes that electrons can change energy levels.

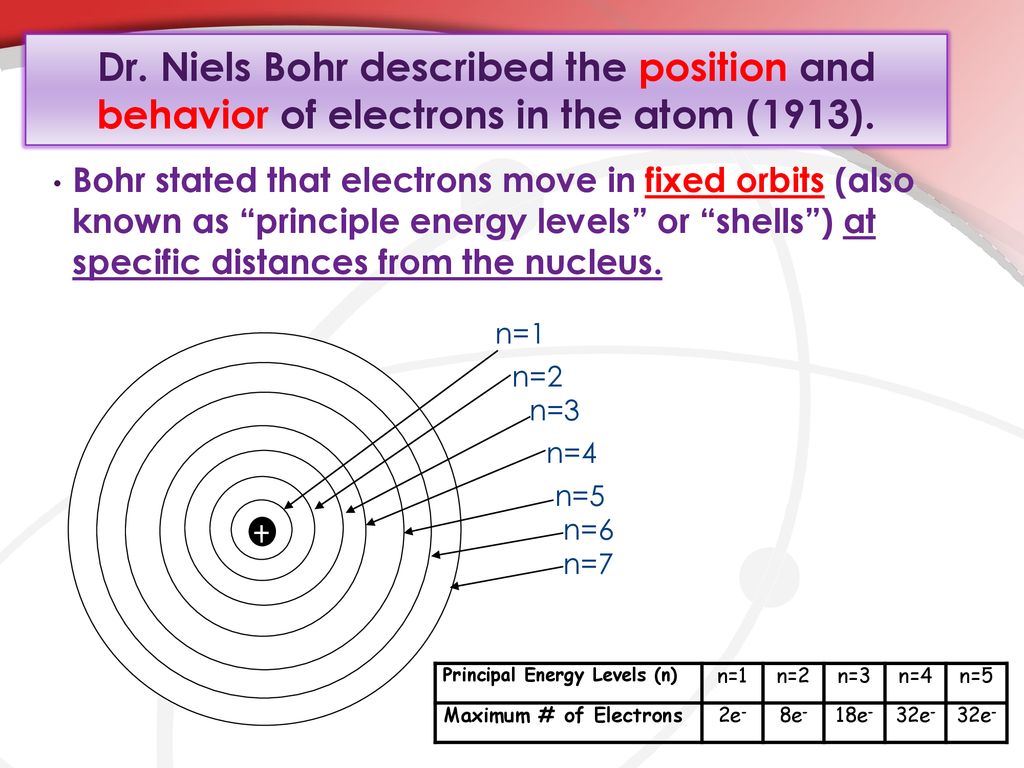
. Neils Bohr proposed that an electron is found only in specific circular paths or orbits around the nucleus. Level 2 to level 4. Energy is transmitted when an electron jumps from one to other orbit near to nucleusand it absorb energy when it jumbs away from nucleushe also said that these electrons are restricted to fixed orbits.
On this page we have gathered for you the most accurate and comprehensive information that will fully answer the question. Explain how Niels Bohrs observation of hydrogens flame test and line spectrum led to his model of the atoms containing electro Inessa 10 Here we need the explain number of line spectrum in hydrogen atom in light of the Bohrs concept of orbit around the nucleus. The electrons revolve in definite orbits that are present around the nucleus.
How did niels bohr describe electrons in his atomic model Answer. Niels Bohr change the atomic theory by realizing that the electrons did not crash into the nucleus as would be expected in classical physics. A hydrogen electron is elevated from level 1 to level 2.
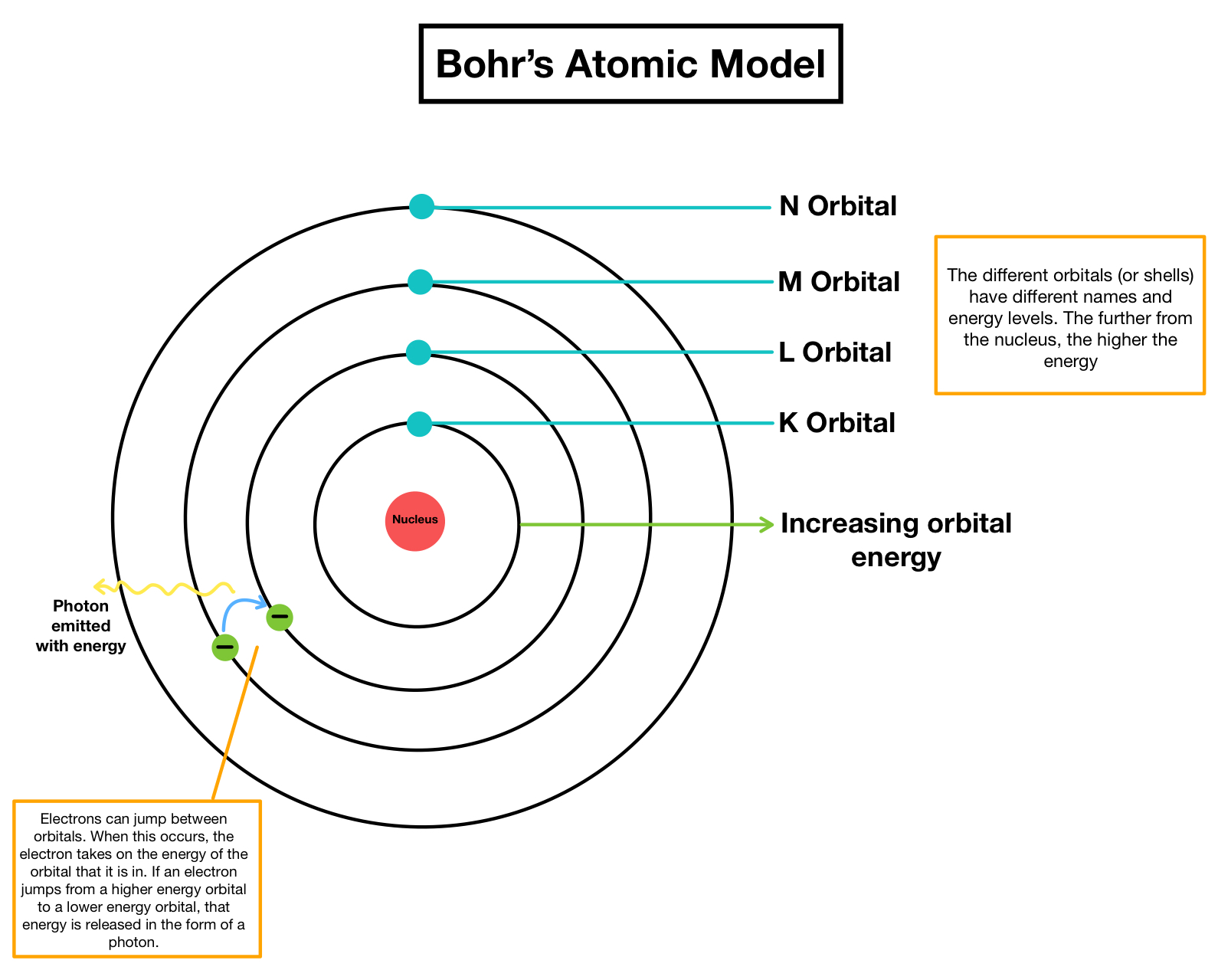
Postulates of Bohrs atomic model. They orbit the central nucleus in discrete paths. But from his special evidence he concluded that electrons are found at only certain distances from the nucleus and have particular values of energy.
So these were called as stationary orbits or stationary states. The transition requiring the greatest energy change is. These orbits were represented either as 1 2 3 or K L M Nand so on.
The energy of an electron is said to be quantized. Bohr thought that electrons orbited the nucleus in quantised orbits. Another electron is elevated from level 2 to level 4.
Energy is transmitted when an electron jumps from one to other orbit near to nucleusand it absorb energy when it jumbs away from nucleushe also said that these electrons are. How did Niels Bohr describe electrons in his atomic model. Shining bright blue light on a strip of metal.
He describe thats electron travel in a circular orbit surrounds the neuclus of an atomeach orbit has quantized energy and size. Light is emitted by electrons when they drop from one energy level to a lower level. The nearest electron to the nucleus has the lowest energy level and the farthest is the strongest energy level.
Level 2 to level 4. The energy of an electron depends on the size of the. In 1913 Bohr proposed his quantized shell mannequin of the atom to clarify how electrons can have steady orbits across the nucleus.
They orbit the central nucleus in discrete pathsAccording to the Bohr model of the atom1. Chemistry 24072020 1701 megankbrown5. 8 How is Bohrs model different from Rutherfords.
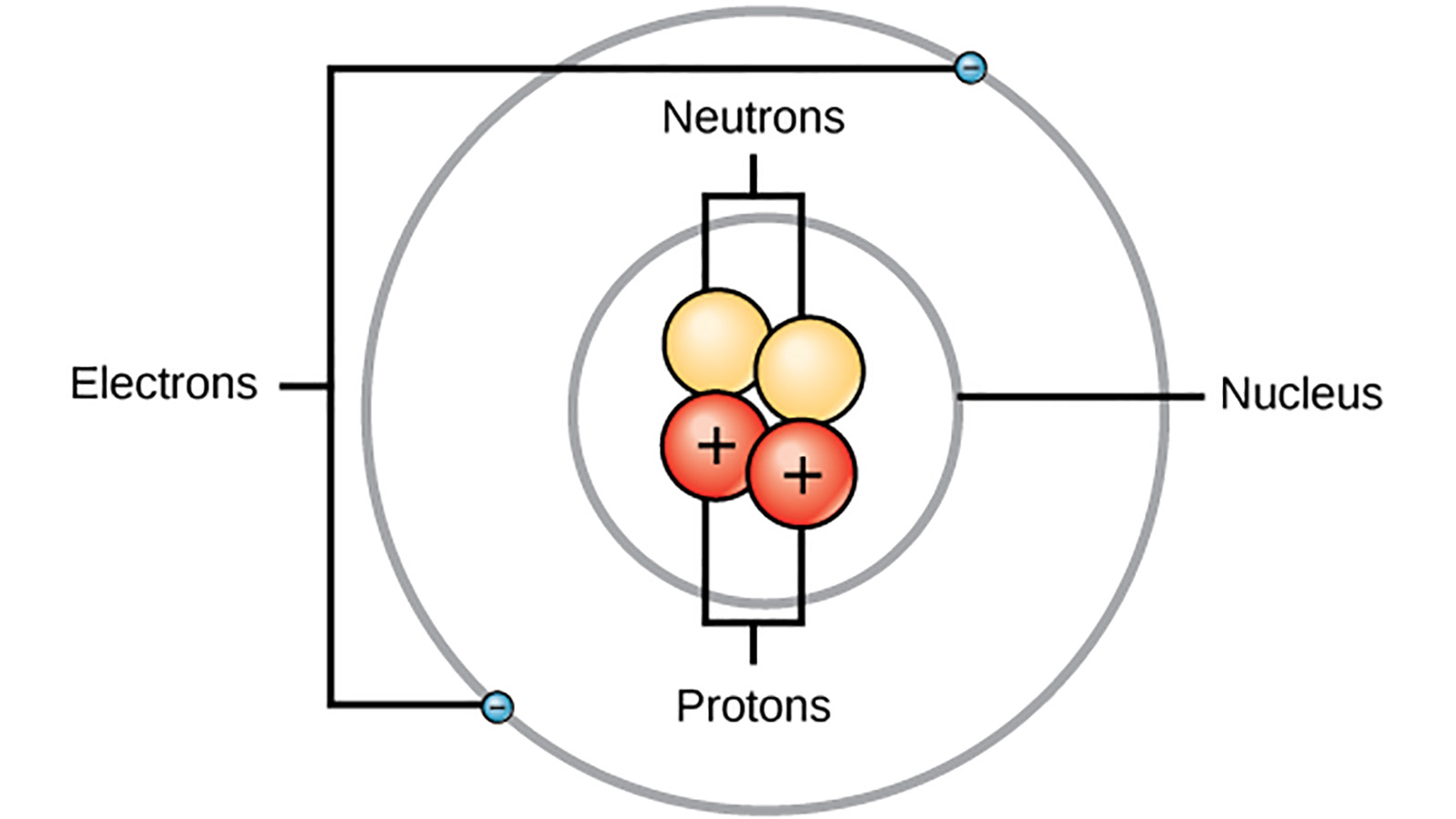
Electrons orbit the nucleus in orbits that have a set size and energy2. Up to 24 cash back Bohr agreed with Rutherfords proposal that in the atom the electrons revolve around a central positively charged nucleus that is responsible for most of the weight of the atom. In 1913 Niels Bohr revised Rutherfords model by suggesting that the electrons orbited the nucleus in different energy levels or at specific distances from the nucleus.
The most appropriate statement that describes the Neils Bohrs atomic model is 1. Radiation can happen solely when the electron jumps from one orbit to a different. 9 What 3 things did Bohrs model propose about electrons.
Light is emitted by electrons when they drop from one energy level to a lower level. Bohr built upon Rutherfords model of the atom. According to the Bohr model of an atom what happens when an electron moves from the second energy level to the third energy level and then back to the second energy level.
So C would seems to be rightExplanation. Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. Neil Bohr describe electrons in his atomic model by saying they orbit the central nucleus in discrete paths.
The Bohr Model explains to us that electrons or negative charges orbit around the atoms nucleus in energy levels. 11 Why is Dalton important with regard to atomic theory. 7 Why was the Bohr theory of the atom developed.
Neils bohar proposed his atomic model in 1913. 10 What was the model of the atom he proposed in 1904 what was the nickname of this model. 12 How did Niels Bohr change the.
Moreover how did Niels Bohr describe electrons in his atomic model. How did niels bohr describe electrons in his atomic model. How did Niels Bohr describe electrons in his atomic model.
Bohr proposed that electrons were in energy levels ground state and absorbed photons of certain frequencies to move to a. Write a balanced. How did niels bohr describe electrons in his atomic model.
He describe thats electron travel in a circular orbit surrounds the neuclus of an atomeach orbit has quantized energy and size. How did niels bohr describe electrons in his atomic model Answer is. Likewise why was Bohrs theory accepted.
Each orbit has a fixed energy and are called energy levels. He believed that electrons moved around the nucleus in circular orbits with quantised potential and kinetic energies. Shining bright blue light on a strip of metal.
How did Niels Bohr describe electrons in his atomic model. A hydrogen electron is elevated from level 1 to level 2. Orbit the nucleus in specific defined paths.
They orbit the central nucleus in discrete paths. The power of an electron will depend on the scale of the orbit and is decrease for smaller orbits. How did niels bohr describe electrons in his atomic model.
When H2g reacts with O2g to form H2Og 242 kJ of energy are evolved for each mole of H2g that reacts. Looking for an answer to the question. The electrons revolve in.
Another electron is elevated from level 2 to level 4. Electrons neither gain energy nor lose energy as long as it is present in a specific orbit. You might be interested in.

3 El Descubrimiento Del Electron Y Del Nucleo Articulo Khan Academy Nuclear Energy Chemical Energy Atomic Structure
How Did Bohr S Model Explain Why The Electron Didn T Spiral Into The Nucleus Quora

Bohr Model Of The Atom Chemtalk

Niels Bohr Kids Britannica Kids Homework Help
What Were Niels Bohr Contribution To Atomic Structure Quora

The Bohr Atom Youtube Niels Bohr Bohr Model Apologia Chemistry

Bohrs Atomic Model Electrical4u

Bohr Atomic Model Postulates Distribution Of Electrons Videos Examples

Niels Bohr Physicist Who Took A Quantum Leap Niels Bohr Atomic Model Bohr Atomic Model

Aim How Did Niels Bohr Describe Electrons In The Atom Ppt Download

Bohrs Atomic Model And Its Limitations

Elektrichestvo Plum Pudding Model Atomic Theory Chemistry Activities

Atomic Theory Ii Chemistry Visionlearning Atomic Theory Vintage Scrapbook Paper Atom Model

Bohr Model Wikipedia The Free Encyclopedia Bohr Model Atom Model Project Atom Model

Niels Bohr Atomic Theory And Its Limitations Atomic Theory Niels Bohr Chemistry Lessons
The Bohr Model Quickly Replaced But Never Forgotten Howstuffworks
Aim How Did Niels Bohr Describe Electrons In The Atom Ppt Download

What Is An Atom Http Sibeda Com What Is An Atom Atom Model Project Neutrons Protons

Comments
Post a Comment